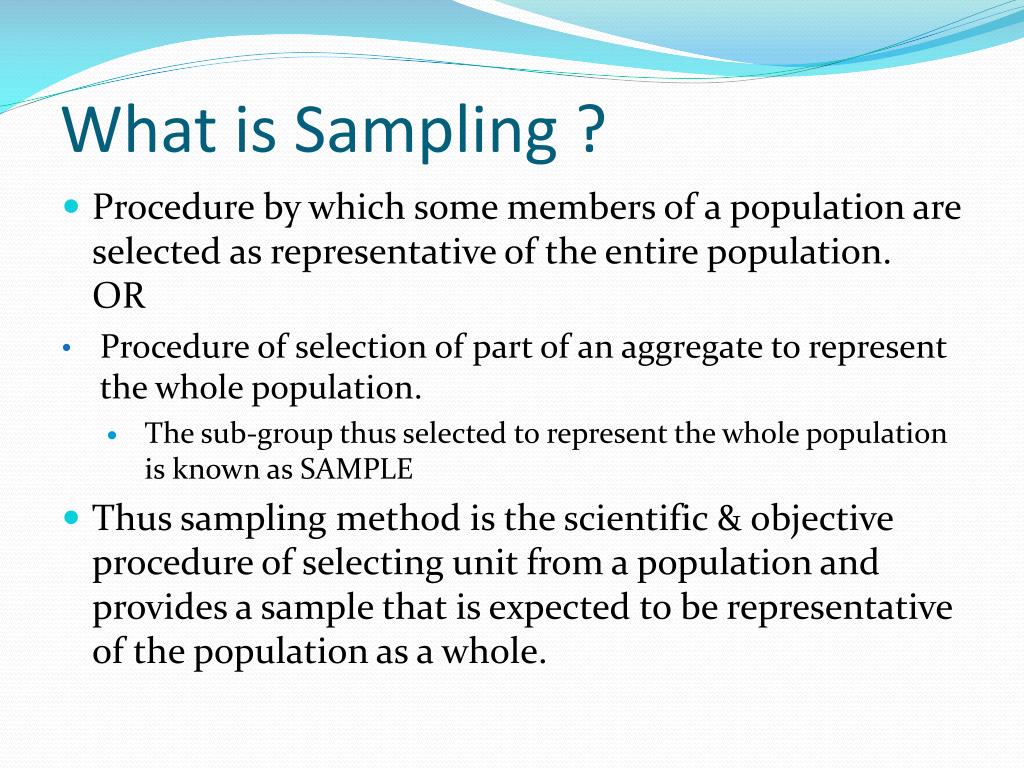
Device Sampling Definition . sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. What they are & when to use which. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical. Variable testing for device functionality: Select the table based upon how sure you want to be about what is observed. guest column | november 24, 2021. Collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures. — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. — sampling plan instructions. — systematic sampling is a probability sampling method in which researchers select members of the.
from www.slideserve.com
— sampling plan instructions. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. Collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures. — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. — systematic sampling is a probability sampling method in which researchers select members of the. guest column | november 24, 2021. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical. What they are & when to use which. Variable testing for device functionality: Select the table based upon how sure you want to be about what is observed.
PPT Sampling Techniques PowerPoint Presentation, free download ID1677543
Device Sampling Definition Collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical. guest column | november 24, 2021. What they are & when to use which. Variable testing for device functionality: — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. — systematic sampling is a probability sampling method in which researchers select members of the. Select the table based upon how sure you want to be about what is observed. Collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures. — sampling plan instructions.
From www.slideserve.com
PPT Sampling PowerPoint Presentation, free download ID1875105 Device Sampling Definition sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. — systematic sampling is a probability sampling method in which researchers select members of the. — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. Regulation (eu) 2017/745. Device Sampling Definition.
From www.slideserve.com
PPT PROBABILITY SAMPLING CONCEPTS AND TERMINOLOGY PowerPoint Presentation ID1273863 Device Sampling Definition — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical. guest column | november 24, 2021. — sampling plan instructions. Select the table based upon how sure you want to be. Device Sampling Definition.
From www.cuemath.com
Methods of Sampling Types, Techniques, Examples Device Sampling Definition — sampling plan instructions. What they are & when to use which. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical. Variable testing for device functionality: — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. Collection of sampling plans, or. Device Sampling Definition.
From educarepk.com
Sampling Techniques Definition, Types & Examples Educare We Educate, We Care. Device Sampling Definition Collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures. guest column | november 24, 2021. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical. Select the table based upon how sure you want to be about what is observed. sampling. Device Sampling Definition.
From www.researchgate.net
Sampling device. Twodimensional device used for sampling consisting of... Download Scientific Device Sampling Definition — systematic sampling is a probability sampling method in which researchers select members of the. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. Select the table. Device Sampling Definition.
From www.knowledgehut.com
What are Sampling Techniques? Different Types and Methods Device Sampling Definition — systematic sampling is a probability sampling method in which researchers select members of the. Select the table based upon how sure you want to be about what is observed. — sampling plan instructions. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical. Variable testing for device functionality: Collection of sampling. Device Sampling Definition.
From www.researchgate.net
Sampling room and sampling device positioning. Download Scientific Diagram Device Sampling Definition — sampling plan instructions. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. What they are & when to use which. Variable testing for device functionality: Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical. Collection of sampling plans, or of. Device Sampling Definition.
From www.slideserve.com
PPT SAMPLING TECHNIQUES PowerPoint Presentation, free download ID2042021 Device Sampling Definition sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. guest column | november 24, 2021. Collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures. Variable testing for device functionality: What they are & when to use which.. Device Sampling Definition.
From www.slideshare.net
Sampling Design Device Sampling Definition Variable testing for device functionality: — sampling plan instructions. — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. What they are & when to use which. Collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures. . Device Sampling Definition.
From www.slideserve.com
PPT Sampling PowerPoint Presentation, free download ID979523 Device Sampling Definition — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical. guest column. Device Sampling Definition.
From thirdspacelearning.com
Types Of Sampling Methods Steps, Examples & Worksheet Device Sampling Definition sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. guest column | november 24, 2021. What they are & when to use which. — systematic sampling. Device Sampling Definition.
From www.slideserve.com
PPT Fundamentals of Sampling Method PowerPoint Presentation, free download ID791407 Device Sampling Definition — sampling is a process in statistical analysis where researchers take a predetermined number of observations from a larger population. — systematic sampling is a probability sampling method in which researchers select members of the. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. Collection of sampling. Device Sampling Definition.
From www.slideshare.net
Sampling1[1] Device Sampling Definition guest column | november 24, 2021. Collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures. Variable testing for device functionality: Select the table based upon how sure you want to be about what is observed. What they are & when to use which. — sampling is a. Device Sampling Definition.
From www.slideshare.net
An overview of sampling Device Sampling Definition Collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. Variable testing for device functionality: What they are & when to use which. guest column | november 24, 2021.. Device Sampling Definition.
From www.intellspot.com
Types of Sampling Methods in Research Briefly Explained Device Sampling Definition — systematic sampling is a probability sampling method in which researchers select members of the. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. — sampling is a process in statistical. Device Sampling Definition.
From www.researchgate.net
Description of the sampling devices Download Scientific Diagram Device Sampling Definition — systematic sampling is a probability sampling method in which researchers select members of the. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. guest column | november 24, 2021. — sampling is a process in statistical analysis where researchers take a predetermined number of observations. Device Sampling Definition.
From researchmethod.net
Sampling Methods Types, Techniques and Examples Device Sampling Definition Select the table based upon how sure you want to be about what is observed. guest column | november 24, 2021. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition, see glossary) for a. Variable testing for device functionality: — sampling plan instructions. What they are & when to use which.. Device Sampling Definition.
From thirdspacelearning.com
Systematic Sampling Steps, Examples & Worksheet Device Sampling Definition Collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures. What they are & when to use which. — systematic sampling is a probability sampling method in which researchers select members of the. sampling comprises the operations designed to select a portion of a pharmaceutical product (for definition,. Device Sampling Definition.